Researchers taking part in a project supported by the Austrian Science Fund FWF, in cooperation with a team of researchers from the Weizmann Institute of Science in Israel and Drexel University in Philadelphia, US, have shown that lead-halide perovskite materials' instability can be considerably reduced through high levels of doping with chloride ions.
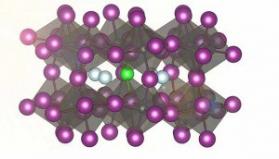
The scientists discovered that certain perovskites can hold high levels of chloride ions, and that this enhances the stability of the functional material under certain conditions by up to two orders of magnitude. They examined cesium-lead-iodide perovskites, where a known issue is the stability of the functional phase of this material - under certain conditions, a phase transition occurs and the excellent photovoltaic properties are lost almost immediately. Previous experiments on perovskites (including chloride instead of iodide ions) suggest that doping the material with chloride may enhance its stability. However, achieving this in practice proved to be extremely difficult.
The team chose an interdisciplinary approach to investigate whether chloride doping could have a positive effect on the stability of cesium-based perovskites. They used atomistic simulations to show that chloride ions that are mobile in the perovskite crystal, can easily be incorporated into the host material, and that this would enhance the mechanical stability. The scientists designed an experimental approach to introduce chloride into the perovskite material, which they achieved by using a chemical sintering process.
When analyzing the stability of the cesium-lead-iodide-chloride, the team was surprised. As lead-halide perovskites are typically particularly unstable in contact with water, the team monitored material stability of the new compounds at different levels of humidity. At a relative humidity of 54%, the half-life of the functional phase of the new material was six times longer than that of control samples without chloride. At reduced humidity levels of 11%, the half-life became even longer.