![]() EPFL is a Switzerland-based technical university and research center. EPFL is focuses on three missions: teaching, research and technology transfer. EPFL works together with an extensive network of partners including other universities and institutes of technology, secondary schools and colleges, industry and economy, political circles and the general public.
EPFL is a Switzerland-based technical university and research center. EPFL is focuses on three missions: teaching, research and technology transfer. EPFL works together with an extensive network of partners including other universities and institutes of technology, secondary schools and colleges, industry and economy, political circles and the general public.
EPFL does extensive perovskite R&D work and is responsible for many publications and advancements in the field.
Route Cantonale
1015 Lausanne
Switzerland
The latest EPFL perovskite news:
Researchers demonstrate laser-driven control of fundamental motions of the lead halide perovskite atomic lattice
An international team of scientists from Fritz Haber Institute of the Max Planck Society, École Polytechnique in Paris, Columbia University in New York, and the Free University in Berlin have demonstrated laser-driven control of fundamental motions of the lead halide perovskite (LHP) atomic lattice.
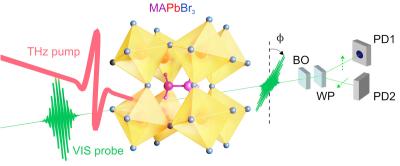
Sketch of the experimental pump-probe configuration. Image from Science Advances
By applying a sudden electric field spike faster than a trillionth of a second (picosecond) in the form of a single light cycle of far-infrared Terahertz radiation, the team unveiled the ultrafast lattice response, which might contribute to a dynamic protection mechanism for electric charges. This precise control over the atomic twist motions could allow to create novel non-equilibrium material properties, potentially providing hints for designing the solar cell material of the future.
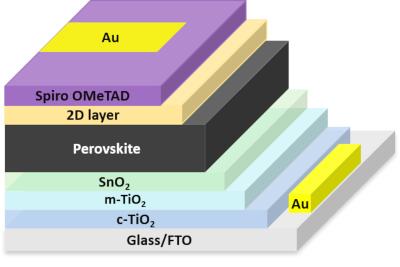
Researchers develop 3D/2D perovskite solar cell with 23.08% efficiency thanks to PEAI salt
An international research team that includes scientists from EPFL in Switzerland, Middle East Technical University (METU) in Turkey, Lomonosov Moscow State University in Russia and The University of Tokyo has fabricated a quasi-2D perovskite solar cell with a unique type of salt to enhance hole extraction.
The triple-cation perovskite absorber was treated with phenethylammonium iodide (PEAI), a modulator that alters the perovskite film's surface energy and forms a quasi-2D structure without further annealing. The result is a 23.08%-efficient device that is also able to retain 95% of its initial efficiency after 900 hours.
Researchers report triple junction perovskite solar cell with 24.3% efficiency
Researchers from the University of Toronto in Canada, Northwestern University, The University of Toledo and University of North Carolina at Chapel Hill in the United States, King Abdullah University of Science and Technology (KAUST) in Saudi Arabia, Yunnan University in China, Ecole Polytechnique Fedérale de Lausanne (EPFL) in Switzerland and University of Warwick in the UK have developed a triple-junction perovskite solar cell with a record efficiency of 24.3% with an open-circuity voltage of 3.21 V.
The NREL has certified the cell’s quasi-steady-state efficiency as 23.3%, which the team stated is the first reported certified efficiency for perovskite-based triple-junction solar cells. They added that triple-junction perovskite solar cells have so far demonstrated a maximum efficiency of around 20%.
EPFL-led team uses additives to improve the stability and efficiency of perovskite solar cells
A team of researchers, led by Professor Michael Grätzel at EPFL and Xiong Li at the Michael Grätzel Center for Mesoscopic Solar Cells in Wuhan (China), have developed a technique that addresses stability concerns of perovskite solar cells (PSCs) and increases their efficiency.
The researchers introduced a phosphonic acid-functionalized fullerene derivative into the charge-transporting layer of the PSC as a “grain boundary modulator”, which helps strengthen the perovskite crystal structure and increases the PSC’s resistance to environmental stressors like heat and moisture.
Researchers turn to facet engineering for more stable perovskite solar cells
Researchers at Switzerland's EPFL and Sungkyunkwan University in Korea have addressed the issue of perovskite solar cells' stability. They focused on the degradation of perovskite thin films, which can be damaged by exposure to moisture, heat, and light. The team looked at two specific crystal facets (the crystal's flat surface), characterized by a particular arrangement of atoms. The arrangement of atoms on these facets can affect the properties and behavior of the crystal, such as its stability and its response to external stimuli like moisture or heat.
The researchers looked at the (100) and (111) facets of perovskite crystals. The (100) facet is a plane that is perpendicular to a crystal's c-axis with its atoms arranged in a repeating pattern in the form of a square grid. In the (111) facet the atoms are arranged in a triangular grid. The study found that the (100) facet, which is most commonly found in perovskite thin films, is particularly prone to degradation as it can quickly transition to an unstable, inactive phase when exposed to moisture. In contrast, the (111) facet was found to much more stable and resistant to degradation.
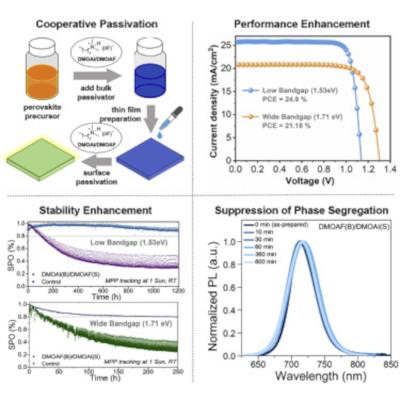
EPFL team uses unique modulators to improve the efficiency and stability of perovskite solar cells
A team of researchers at EPFL have developed a method that improves both power conversion efficiency and stability of solar cells based on pure iodide as well as mixed-halide perovskites. The new method aslo suppresses halide phase segregation in the perovskite material. The research was carried out by the groups of Professors Michael Grätzel and Ursula Rothlisberger at EPFL and led by Dr Essa A. Alharbi and Dr Lukas Pfeifer.
The method treats perovskite solar cells with two alkylammonium halide modulators that work synergistically to improve solar cell performance. The modulators were used as passivators, compounds used to mitigate defects in perovskites, which are otherwise promoting the aforementioned degradation pathways.
Researchers achieve high-performance flexible all-perovskite tandem solar cell
Researchers from Empa, EPFL, Sichuan University, Jiaxing University, Soochow University, University of Cologne, University of Potsdam, HZB and the University of Oxford have developed a flexible all-perovskite tandem solar cell with a mitigated open-circuit voltage deficit and reduced voltage loss. The team reported flexible tandem efficiency of almost 24% on small area cells using the spin coating method.
Flexible all-perovskite tandem cells are currently less developed than rigid cells, due to a difficult deposition process for the cell's functional layers and a lower open-circuit voltage. This is due to high defect densities within the perovskite absorber layer and at the perovskite/charge selective layer interface.
Researchers perform design and cost analysis of 100 MW perovskite solar panel manufacturing process in different locations
Scientists from Switzerland's EPFL and the Toyota Motor Corporation have prepared a detailed analysis of the projected costs of designing and operating a 100 MW perovskite solar cell production line in various locations, taking under consideration factors like labor and energy costs as well as all materials and processing. The team found that perovskite PV could be cost-competitive with other technologies even at much smaller scale, but noted that this still depends on the tech proving its long-term stability, and impressive achievements in research being successfully transferred to commercial production.
While perovskite materials have been repeatedly demonstrating their potential for low-cost, high-efficiency solar energy with lower energy fabrication compared to silicon PV technology, there are still many different possibilities regarding the form these commercial products could take, and the materials they could contain - with more than one option that could prove commercially viable.
Researchers achieve 18.4% efficiency for 4T flexible perovskite-CIGS tandem mini-module
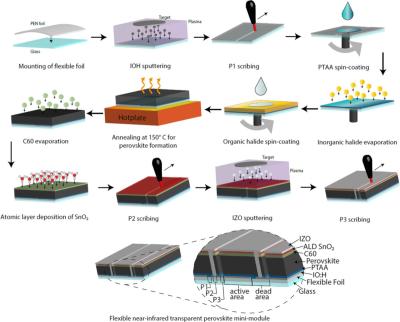
Researchers from Switzerland's Federal Laboratories for Materials Science and Technology (EMPA) and École Polytechnique Fédérale de Lausanne (EPFL) have designed a four-terminal tandem mini-module based on perovskite and copper, indium, gallium and selenium (CIGS) with an aperture area of around 2 cm2, and a geometric fill factor of over 93%.
Processing sequence of flexible NIR-transparent perovskite mini-module. Image from RRL Solar
The team reports that the key to efficient flexible perovskite-CIGS tandem modules is the development of near-infrared (NIR) transparent perovskite solar modules on a flexible polymer foil. To achieve these results, the researchers had to overcome the challenges of laser patterning on flexible substrates to realize the first all-laser scribed monolithically interconnected NIR-transparent perovskite mini-modules on polymer film. The perovskite mini-module used in the tandem panel was fabricated on a flexible polyethylene napthalathe (PEN) substrate mounted to a glass substrate in a p–i–n device architecture. This configuration, according to the research team, shows reduced absorption in the NIR region.
Researchers from EPFL and CSEM achieve 31.25% efficiency for tandem perovskite-silicon solar cell
Researchers from the Swiss Center for Electronics and Microtechnology (CSEM) and the École polytechnique fédérale de Lausanne (EPFL) have reported a power conversion efficiency exceeding 30% for a 1 cm2 tandem perovskite-silicon solar cell, which represents a world record for a PV device of this kind. The team achieved an efficiency of 30.93% for a 1 cm2 solar cell based on high-quality perovskite layers from solution on a planarized silicon surface and an efficiency of 31.25% on a cell of the same size and fabricated with a hybrid vapor/solution processing technique compatible with a textured silicon surface.
“These results constitute two new world records: one for the planar and one for the textured device architecture,” the researchers explained, noting that both efficiencies were certified by the US Department of Energy's National Renewable Energy Laboratory (NREL). “The latter approach provides a higher current and is compatible with the structure of current industrial silicon solar cells”. Unfortunately, the researchers did not disclose technical details on how they improved the efficiency of both devices.
Pagination
- Previous page
- Page 2
- Next page