Researchers at MIT have developed a simple procedure for making a perovskite solar cells using lead recovered from lead-acid car batteries, a method that could have both environmental and health benefits.
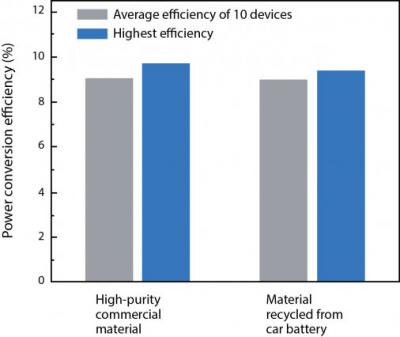
They first drained and disassembled the batteries, then extracted the lead from the anode and lead dioxide from the cathode; the latter was then processed to make lead oxide. Both were then dissolved in acids and mixed with aqueous potassium iodide. The lead iodide precipitate is dried to a powder, dissolved in solvent and spin coated onto a substrate. The lead iodide precipitate was dried to a powder, dissolved in solvent and spin coated onto a substrate. The lead iodide film then went through a reaction with organic halide to form perovskite.
While lead is toxic, it can be encapsulated in other materials to prevent environmental contamination and still remain recoverable. Even so, the MIT team is also investigating alternatives to the use of lead in high efficiency perovskite solar cells.
Source: energymatters

