Scientists at Syracuse University and Brookhaven National Laboratory found a new way to visualize and monitor chemical reactions in real time using perovskites. They have designed a nanomaterial that changes color when it interacts with ions and other small molecules during a chemical reaction, allowing to monitor reactions qualitatively with the naked eye and quantitatively with simple instrumentation.
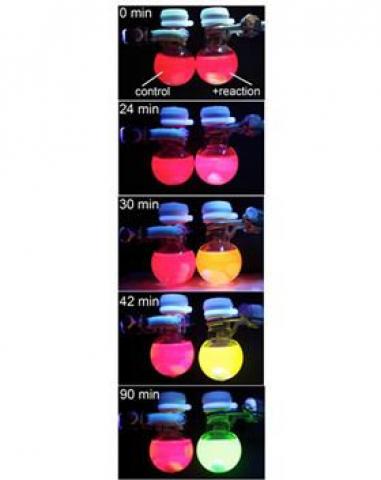
The researchers explain that many chemical reactions occur in a solution that is colorless and transparent so the only way to know if a reaction has occurred or not is to perform extensive analysis after a multi-step purification. This new method represents a simpler way to investigate why and how fast a reaction occurs (if at all). The group has designed a nanoparticle that reacts with by-products of the reaction. When the reaction occurs, the nanoparticle fluoresces at a different color, allowing to gauge kinetics by eye, instead of using special equipment.
The team used perovskites that are composed of metal ions and a halide (a bromine, chlorine or iodine ion). At the nanoscale, perovskites are photo-luminescent, meaning they emit light when 'excited' by a laser or lamp. The fact that the colors they emit are determined, in part, by their ion concentrations makes perovskites unique among nanomaterials. It also makes them suitable for applications like solar cells, light-emitting diodes, lasers and photo detectors.
The scientists used the ion concentration ratios of perovskites to detect ions in solution, and then monitor the chemical reaction. They started by working with a simple system that involved organic reactions of molecules called organohalides. When these molecules react, often forming carbon-carbon double bonds in what is known as an elimination reaction, they release a halide. Typically, the halide is an unimportant side-product of the reaction, until now.
This technology allows to accurately detect the halide release. When the reaction starts, the perovskite fluoresces bright red. As the halide is released, or exchanged in the chemical reaction, the particle absorbs it, and the fluorescence color changes proportionally to the halide concentration ' from red to orange to yellow to green. When the color is green, the reaction is over. Added to that is the fact that the perovskite concentration is very low - it's only necessary to add a small amount to the reaction for observation.
The team stated that it is now able to measure very precise chemical kinetics by monitoring the color change with nothing more than an ultraviolet lightbulb or a simple fluorescence spectrometer. The group's technology is patent-pending at Syracuse University and they are now testing the technique's applicability for a wide range of chemical reactions and its effectiveness at measuring low concentrations of ions and reactive molecules.

