Researchers at the Moscow State University named after MV Lomonosov, in cooperation with scientists from the Kurchatov Synchrotron Radiation Center, explained the key mechanisms of interaction of hybrid perovskites with solvents and based on the results obtained, suggested new approaches to obtaining a perovskite light-absorbing layer of thin-film solar cells from weakly coordinating aprotic solvents.
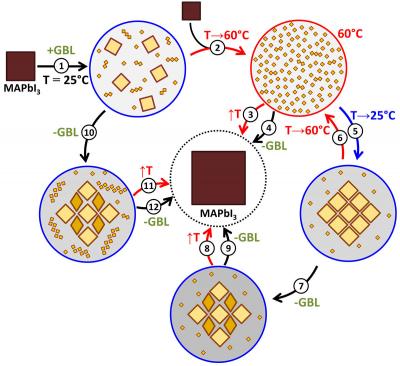 The scheme of transformations of perovskite components in solution proposed by the authors of the study
The scheme of transformations of perovskite components in solution proposed by the authors of the study
To apply thin films of perovskite from solutions, two solvents are usually used: dimethylsulfoxide and dimethylformamide. However, earlier work of MSU scientists showed that crystallization from them proceeds through the formation of intermediate compounds ' crystal solvates, which can degrade the morphology and functional properties of the perovskite layer.
'We have established that perovskite dissolves at room temperature with the formation of such clusters, and upon heating they decompose to small-sized complexes. This leads to supersaturation and precipitation of perovskite from solution in the form of single crystals. We showed that it was the deposition of the cluster adduct in place of perovskite that prevented the formation of thin films from this solvent and on the basis of an understanding of the processes that occur during the dissolution of perovskite in the lactone, we proposed approaches that direct the crystallization of perovskite around the formation of clusters, which for the first time made it possible to obtain qualitative films . This is an excellent example of the practical application of fundamental chemical knowledge for solving applied material science problems ' just what is generally called fundamental material science throughout the world, 'comments the research team.