Researchers from the University of California San Diego, King Abdullah University of Science and Technology and the Air Force Research Laboratory have developed a technique that could enable the fabrication of longer-lasting and more efficient perovskite solar cells, photodetectors, and LEDs.
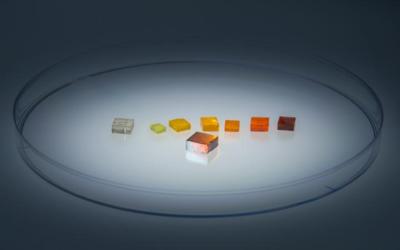 Strain-engineered, single crystal thin film of perovskite grown on a series of substrates with varying compositions and lattice sizes. Image Credit: David Baillot/UC San Diego Jacobs School of Engineering.
Strain-engineered, single crystal thin film of perovskite grown on a series of substrates with varying compositions and lattice sizes. Image Credit: David Baillot/UC San Diego Jacobs School of Engineering.
A major obstacle is the tendency of one of the best-performing perovskite crystals, α-formamidinium lead iodide (HC(NH2)2PbI3, known as α-FAPbI3), to assume a hexagonal structure at room temperature, in which photovoltaic devices are required to operate. This hexagonal structure cannot respond to most of the frequencies of light in solar radiation, and is hence not useful for solar applications as it could be. The team therefore set out to stabilize the structure of α-FAPbI3, using a simple but useful approach known as strain engineering, which has been used to tune the electronic properties of semiconductors.
One of the most interesting but problematic features of halide perovskites is that only a few, including α-FAPbI3, have high charge mobility and absorb light strongly, but the technologically useful crystal structures of these few compounds are unstable ' some can spontaneously transform into other phases in less than a second. In photovoltaic devices, fast-moving charge carriers and strong light absorption are both vital in order to convert solar energy into electricity with high efficiency. Unfortunately, structural stability, high charge mobility and the ability to absorb light strongly don't seem to readily coexist in perovskite halides.
In this work, the team used strain engineering to tackle this problem. They grew crystalline α-FAPbI3 from a solution so that it formed on another, more stable halide perovskite (the substrate). The FAPbI3 atoms in the growing crystal align with the cubic structure of the atoms in the substrate, thereby forming a pseudocubic structure themselves. The alignment of atoms in different materials is called epitaxy. This epitaxy locks α-FAPbI3 into the pseudocubic structure as a result of the strong chemical forces between it and the substrate, preventing its transformation into the undesirable hexagonal structure. The authors find that the pseudocubic structure remains stable for at least a year at room temperature.
The researchers were able to control the strain of α-FAPbI3 from 0 to 2.4% compressive deformation by growing FAPbI3 on substrates that have different lattice dimensions. This recent study provides an extremely accessible and practical avenue through which to explore and use the physical properties of strained halide perovskites.
Questions remain, however, as to how the authors' findings could be used in solar cells. Currently available halide perovskite photovoltaic devices do not contain a genuinely epitaxial substrate, and so new cell designs will be needed to make use of the reported discovery. But a range of halide perovskite compounds are available that have similar atomic arrays to α-FAPbI3, and which exhibit many different technologically important electronic properties. This study therefore suggests that there is plenty of scope for designing and developing epitaxial quantum-well devices using these materials, by mimicking the way in which quantum-well devices were developed using semiconductors from the III'V family of materials. This might bring down the cost of manufacturing these devices.
Finally, it will be interesting to see whether crystals of halide perovskites can be grown with sufficient atomic precision to make superlattices ' periodic structures that contain multiple layers of two or more materials. The use of halide perovskites in superlattices could open up otherwise inaccessible electronic band structures, thereby allowing a rich array of physics to be explored, and emerging quantum-well devices to be further developed.

