Researchers at the Tokyo Institute of Technology have developed a barium ruthenium-based (BaRuO3) perovskite catalyst that shows strong activity even at low temperatures (down to 313 K). This is said to be the first catalyst of its kind capable of the selective oxidation of sulfides under mild conditions, with molecular oxygen (O2) as the only oxidant. The reusable catalyst does not require additives, and so can prevent the formation of toxic by-products. The oxidation of sulfides is a commercially important process with broad applications ranging from chemicals production to environmental management.
The researchers state that BaRuO3 has three advantages over conventional catalysts. Firstly, it exhibits high performance even at 313 K, a temperature much lower than the 373-423 K range reported in previous systems including other ruthenium- and manganese-based catalysts. Secondly, its high rate of oxygen transfer indicates that it has many potential uses; for example, it is applicable to the oxidative desulfurization of dibenzothiophene, which can produce a 99% yield of pure sulfone. Thirdly, the new catalyst is recyclable - the present study showed that BaRuO3 could be reused at least three times without loss of performance.
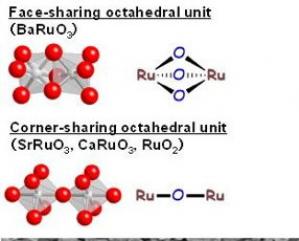
The catalyst has a rhombohedral structure. While other ruthenium-based catalysts investigated to date such as SrRuO3, CaRuO3 and RuO2 can all be described as having corner-sharing octahedral units, BaRuO3 has face-sharing octahedra. This configuration is thought to be one of the main reasons behind the catalyst's higher oxygen transfer capability.
The BaRuO3 material was synthesized based on the sol-gel method using malic acid - also an important factor as the researchers explain: "The catalytic activity and specific surface area of BaRuO3 synthesized by the malic acid-aided method were higher than those of BaRuO3 synthesized by the polymerized complex method."
The study highlights the importance of subtle changes in the nanoscale structure of perovskite catalysts, and could provide promising leads for further research on a wide range of perovskite-based functional materials.