Perovskites are materials that share a crystal structure similar to the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3).
Depending on which atoms/molecules are used in the structure, perovskites can possess an impressive array of interesting properties including superconductivity, ferroelectricity, charge ordering, spin dependent transport and much more. Perovskites therefore hold exciting opportunities for physicists, chemists and material scientists.
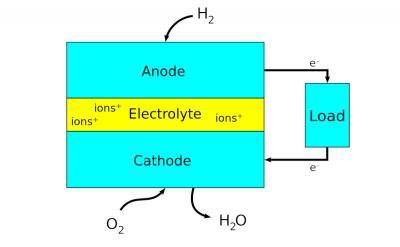
Fuel cells are electrochemical energy conversion devices that produce electricity via chemical reaction. They convert potential chemical energy into electrical energy and generate heat as a by-product. A major advantage of fuel cells is that they are “green” - they generate electricity with very little pollution, as much of the hydrogen and oxygen used in generating electricity ultimately combine to form a harmless byproduct - water.
Fuel cells can be used in a wide range of applications, including transportation, material handling, stationary, portable, and emergency backup power applications. Fuel cells have several benefits over conventional combustion-based technologies currently used in many power plants and passenger vehicles; they can operate at higher efficiencies than combustion engines, and can convert the chemical energy in the fuel to electrical energy with efficiencies of up to 60%. Fuel cells have lower emissions than combustion engines. Hydrogen fuel cells emit only water, so there are no carbon dioxide emissions and negative impact on the environment.
It appears that designing inexpensive, efficient, reliable fuel cells is not such a simple affair.
Scientists have designed many different types and sizes of fuel cells in their search for greater efficiency. A major point is the choice of electrolyte. The design of electrodes, for example, and the materials used to make them, heavily depend on the used electrolyte.
The type of fuel also depends on the electrolyte. Some cells need pure hydrogen, and therefore demand extra equipment such as a "reformer" to purify the fuel. Other cells can tolerate some impurities, but might need higher temperatures to run efficiently. Liquid electrolytes circulate in some cells, which requires pumps. The type of electrolyte also dictates a cell's operating temperature.
Each type of fuel cell has advantages and drawbacks compared to the others, and none of them is currently cheap and efficient enough to widely replace traditional ways of generating power.
Perovskites have been studied for various parts of fuel cells. Components like electrolytes, electrodes and interconnects, have all been targeted as potential beneficiaries of perovskite materials. In SOFCs (solid oxide fuel cells), for example, all components (except for the sealant) can potentially be made of perovskite ceramics. In recent years, a significant amount of time has been invested researching the development of perovskite materials for fuel cells, identifying new mixed conductors and improving the operational performance of existing materials through development of improved cell designs. Hopefully perovskites will be able to improve fuel cell technology so it can be put into widespread use.
Researchers identify the potential of hexagonal perovskite oxides for next-gen protonic ceramic fuel cells
Researchers at Tokyo Institute of Technology and Tohoku University have reported hexagonal perovskite-related oxides with exceptionally high proton conductivity and thermal stability.
The team explained that these materials' unique crystal structure and large number of oxygen vacancies enable full hydration and high proton diffusion, making them ideal candidates as electrolytes for next-generation protonic ceramic fuel cells that can operate at intermediate temperatures without degradation.
Researchers address the challenges of proton-conducting perovskites for next-generation fuel cells
Protonic ceramic fuel cells (PCFCs) have attracted much attention lately. These devices do not operate via the conduction of oxide ions (O2−) but light protons (H+) with smaller valence. A key feature of PCFCs is their ability to function at low and intermediate temperatures in the range of 50–500 °C. However, PCFCs based on perovskite electrolytes reported thus far suffer from low proton conductivity at low and intermediate temperatures.
A research team, led by Professor Masamoto Yashima from Tokyo Institute of Technology (Tokyo Tech), in collaboration with High Energy Accelerator Research Organization (KEK), has set out to address this limitation of perovskite-based proton conductors.
Researchers develop new technique to grow single-crystal perovskite hydrides
Researchers from Japan's Shibaura Institute of Technology and National Institute for Materials Science have developed a method to grow single-crystal perovskite hydrides, enabling accurate hydride conductivity measurements.
Perovskite hydrides, whose molecular structure contains hydrogen anions (H−), attract special attention because of their hydrogen-derived properties and many believe they can be useful for hydrogen storage technologies such as fuel cells and next-generation batteries, as well as energy-saving superconducting cables. However, measuring their intrinsic hydride-ion conductivity is difficult. In their recent study, the researchers addressed this issue using a novel laser deposition technique in an H-radical atmosphere. Using this approach, they grew thin-film single crystals of two different perovskite hydrides and characterized their hydride-ion conductivity.
Researchers design new method to enhance perovskites' oxygen reduction performance in hydrogen fuel cells
Researchers from Huazhong University of Science and Technology, Tohoku University, Lanzhou University, Tsinghua University and Georgia Institute of Technology have reported on a new method to enhance the electrochemical surface area (ECSA) in a calcium-doped perovskite, La0.6Ca0.4MnO3 (LCMO64), which could help in overcoming a common bottleneck in the application of perovskite oxides as electrocatalysts in hydrogen fuel cells.
Perovskite oxides have potential for use in various fields thanks to their interesting and diverse properties. Their high intrinsic activities also position them as a promising alternative to noble metal catalysts for efficiently catalyzing the oxygen reduction reaction (ORR). However, their application is still hampered by their poor electrical conductivity and low specific surface area.
Researchers explore conduction mechanisms in a unique perovskite oxide
Researchers at the Tokyo Institute of Technology (Tokyo Tech), in collaboration with Tohoku University, Australian Nuclear Science and Technology Organization (ANSTO) and the High Energy Accelerator Research Organization (KEK), recently investigated a promising material for next-generation electrochemical devices: hexagonal perovskite-related oxide Ba7Nb3.8Mo1.2O20.1. The team unveiled the material's unique ion-transport mechanisms, that could pave the way for better dual-ion conductors.
Clean energy technologies are the cornerstone of sustainable societies, and solid-oxide fuel cells (SOFCs) and proton ceramic fuel cells (PCFCs) are among the most promising types of electrochemical devices for green power generation. These devices, however, still face challenges that hinder their development and adoption.
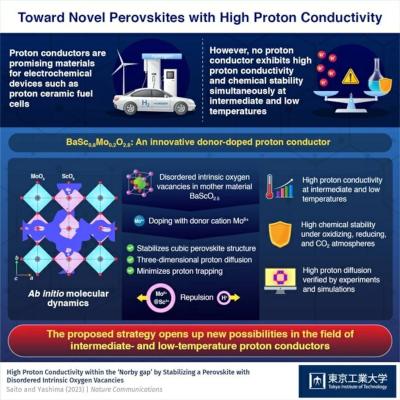
Researchers propose a new perovskite-based strategy that could revolutionize the design and development of proton conductors
Tokyo Institute of Technology researchers have shown that donor doping into a mother material with disordered intrinsic oxygen vacancies, instead of the widely used strategy of acceptor doping into a material without oxygen vacancies, can greatly enhance the conductivity and stability of perovskite-type proton conductors at intermediate and low temperatures of 250–400 ℃, (e.g. 10 mS/cm at 320 ℃). This approach provides a new design direction for proton conductors for fuel cells and electrolysis cells.
Protonic ceramic (or proton conducting) fuel/electrolysis cells (PCFCs/PCECs) are a strong contender for future sustainable energy technologies. These devices can directly convert chemical energy into electricity and vice versa with zero emissions at low or intermediate temperatures, making them an attractive option for many emerging applications such as next-generation distributed power sources. In addition, unlike other types of fuel cells and electrolysers, the PCFCs/PCECs do not require precious metal catalysts or expensive, heat-resistant alloys.
DoE funded project will use PSCs to produce green hydrogen
A Department of Energy (DoE) project, lead by University of Michigan's Prof. Zetian Mi, is using perovskites to develop high efficiency, low cost, and ultrastable production of green hydrogen fuels directly from sunlight and water.
The new method to achieve clean hydrogen through solar water splitting offers a promising path to achieving net-zero carbon emissions. The University of Michigan research team aims to stabilize perovskite-based solar cells to produce highly-efficient, low-cost, ultrastable green hydrogen fuel.
Researchers examine a perovskite-based electrolyte developed for SOFCs
Solid oxide fuel cells, or SOFCs, are a type of electrochemical device that generates electricity using hydrogen as fuel, with the only 'waste' product being water.
To potentially accelerate the development of more efficient SOFCs, a research team from Kyushu University, Yamagata University and Kyushu Synchrotron Light Research Center has uncovered the chemical innerworkings of a perovskite-based electrolyte developed for SOFCs. The team combined synchrotron radiation analysis, large-scale simulations, machine learning, and thermogravimetric analysis, to uncover the active site of where hydrogen atoms are introduced within the perovskite lattice in its process to produce energy.
Researchers use UV light to modulate oxide ion transport in a perovskite crystal at room temperature
Researchers from Japan's Tsukuba University have found that ultraviolet light can modulate oxide ion transport in a perovskite crystal at room temperature.
The performance of battery and fuel cell electrolytes depends on the motions of electrons and ions within the electrolyte. Modulating the motion of oxide ions within the electrolyte could enhance future battery and fuel cell functionality by increasing the efficiency of the energy storage and output. Use of light to modulate the motions of ions - which expands the source of possible energy inputs - has only been demonstrated thus far for small ions such as protons. Overcoming this limitation of attainable ion motions is something the researchers in this study aimed to address.
New data on double perovskite oxides could promote their use in fuel cells
A joint research team that includes researchers from the Institute of Solid State Chemistry and Mechanochemistry (the Ural Branch of the Russian Academy of Sciences), the Donostia International Physics Centre and the HSE Tikhonov Moscow Institute of Electronics and Mathematics has studied the characteristics of cubic double perovskite oxides.
To date, experimental measurements of the minerals' characteristics have not corresponded to the results of theoretical modeling. In this new work, the researchers set out to better understand this disparity. The data obtained could allow the improvement of low-temperature fuel cell technologies'one of the main alternatives to current sources of electricity.
Pagination
- Page 1
- Next page