Perovskites are materials that share a crystal structure similar to the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3).
Depending on which atoms/molecules are used in the structure, perovskites can possess an impressive array of interesting properties including superconductivity, ferroelectricity, charge ordering, spin dependent transport and much more. Perovskites therefore hold exciting opportunities for physicists, chemists and material scientists.
Lead-based perovskites have become key materials in photovoltaics research thanks to their facile solution processability and impressive performance. However, despite their great potential, a persistent threat on future use and commercialization is the issue of toxicity and lead-content.
Lead (and its oxide form) is highly hazardous to animals and humans. Hence, succeeding to develop lead-free perovskites that could be used instead of the lead-based ones is a much desired accomplishment. While producing lead-free perovskite materials is not very hard to do, and various candidates exist (Sn-based perovskites, for example) these tend to perform poorly compared to lead-based perovskite materials, and exhibit limited optoelectronic performance and stability. However, vigorous research is taking place and hopefully it will yield lead-free perovskites that can serve as a viable replacement to lead-based ones.
Researchers develop wearable photoferroelectric perovskite X-Ray detectors
Researchers from China's Shaanxi Normal University, Zhejiang Normal University, China Institute of Radiation Protection and Chinese Academy of Sciences have developed lead-free photoferroelectric hybrid metal halide perovskite flexible membranes for wearable detectors, that offer excellent X-ray response with high sensitivities, low detection limit and impressive imaging capabilities.
Demonstration and application potential for lead-free photoferroelectric perovskite membrane (LFPPM). a) Optical images of the LFPPM. b) Schematic diagram of LFPPM wearable X-ray dosimeter. c) Schematic diagram of the working principle of wearable X-ray dosimeter. (Image credit: Nanowerk)
High-sensitivity wearable radiation detectors are important for personnel protection in radiation environments such as defense, nuclear facilities, and medical fields. Traditional detectors using bulk crystals tend to lack flexibility. Hybrid metal halide perovskites have shown promise for next-generation radiation detection as they can efficiently absorb high-energy radiation and convert it into electrical signals. However, there are concerns of lead toxicity. More recent efforts have explored lead-free alternatives, but these have generally suffered from poor charge transport properties, reducing their effectiveness as radiation detectors.
Researchers develop new encapsulation strategy based on shellac
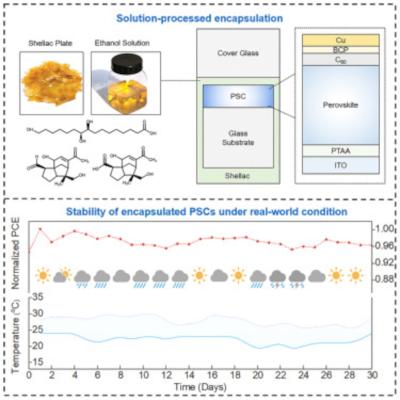
Researchers at the Chinese Academy of Sciences (CAS), Shanghai Jiao Tong University School of Medicine and the University of Electronic Science and Technology of China (UESTC) have presented a simple and economical encapsulation strategy with shellac to protect perovskite solar cells (PSCs) under various accelerated degradation experiments.
The shellac-encapsulated (SE) PSC modules reportedly passed outdoor stability, UV preconditioning, and hail tests according to the International Electrotechnical Commission 61215 standard (IEC61215).
Researchers design “cage traps” for lead management of perovskite solar cells
Researchers from Zhengzhou University and the Chinese Academy of Sciences (CAS) have devised a novel lead capturing technique for perovskite solar cells: they implanted a multifunctional mesoporous amino-grafted-carbon net into the perovskite solar cells, creating biomimetic cage traps that could effectively mitigate Pb leakage and shield from external invasion under extreme weather conditions.
The team then explored the synergistic Pb capturing mechanism in terms of chemical chelation and physical adsorption. Additionally, the Pb contamination assessment of end-of-life perovskite solar cells in the real-world ecosystem, including Yellow River water and soil, was proposed by the scientists.
Researchers design titanium dioxide sponge to prevent lead leakage in perovskite solar cells
Researchers from CNR-IMM,CNR-IPCB, CNR-NANOTEC, Università Degli Studi di Messina and the University of Basel have shown that lead leakage can be prevented by applying a transparent titanium dioxide (TiO2) sponge in a semitransparent solar cell. The device has demonstrated comparable efficiency to semi-transparent perovskite devices and has an average visible transmittance (AVT) of 31.4%.
The team designed the solar cell for applications such as building-integrated photovoltaics (BIPV) and agrivoltaics, in which the potential lead leakage can be seen as a serious public environmental and health risk source.
Researchers fabricate flexible perovskite solar cells via blade coating in ambient conditions without using toxic solvents
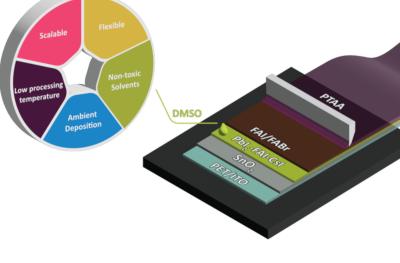
Researchers at University of Rome “Tor Vergata”'s CHOSE (Centre for Hybrid and Organic Solar Energy) and CNR-ISM Institute of Structure of Matter have deposited flexible perovskite solar modules without using toxic solvents, via blade coating in ambient air. 14% PCE was reportedly obtained through the optimization of coating parameters and the use of additives.
The scalable ambient air deposition of perovskite solar devices remains a major challenge of this technology. In addition, toxic solvents are regularly used in perovskite layer deposition, which can damage the environment and endanger the safety of potential production lines. In this recent work, the team managed to address these issues and fabricate sustainable flexible perovskite solar modules (flex-PSMs), in which all layers were deposited via a blade coating in ambient air without the usage of toxic solvents.
Researchers examine perovskite solar cells' toxicity and suggest risks may be overestimated
Scientists from Skoltech (Skolkovo Institute of Science and Technology), Research Centre for Medical Genetics, Federal Research Center of Problems of Chemical Physics and Medicinal Chemistry and Zhengzhou Research Institute of HIT have studied the toxicity of materials used in perovskite solar cells.
They concluded that once the remaining technological hurdles are overcome, mass production of this potentially cheap and efficient alternative to silicon-based photovoltaics should not cause any significant environmental risks and health hazards. The study draws attention to perovskite components other than lead, suggesting that metal's toxicity, by comparison, could be overestimated.
ZSW designs new process for perovskite solar cell production using environmentally friendly precursor solvents
The Centre for Solar Energy and Hydrogen Research Baden-Württemberg (ZSW) has made progress towards the goal of coating large-area perovskite solar cells on an industrial scale using a process that uses more benign solvents than available hazardous solvents like dimethylformamide. The team of researchers developed a coating process for perovskites that uses a single ecofriendly solvent, dimethyl sulfoxide. The ZSW team applied this method to produce a solar cell nearly as efficient as cells made with the toxic solvent.
Perovskite precursors have to first be dissolved so they can be applied in uniform layers to the substrate. This requires solvents that usually contain dimethylformamide (DMF), which is hazardous to health and the environment. This toxicity hampers efforts to scale this process up to industrial production. Manufacturers would have to produce and dispose of larger quantities of the solvent and take even more stringent occupational safety measures, also causing costs to rise. For these reasons, many researchers and manufacturers are in search of environmentally compatible solvents that are suitable for industrial applications. This use case requires chemical properties that very few substances exhibit. Dr. Jan-Philipp Becker, the head of the ZSW’s Photovoltaics: Materials Research department, worked with his team to investigate pure dimethyl sulfoxide (DMSO) to see if it could serve this purpose.
Researchers develop Hybrid 2D/3D Structure for Sn-based metal halide perovskites
Tin (Sn)-based metal halide perovskites (MHPs) could be an environmentally benign alternative to lead-based ones, which are toxic. However, some critical issues need to be resolved before Sn-based MHPs can be leveraged in planar semiconductor devices. When arranged into a 2D structure (or quasi-2D structure with a few layers), defects in the crystal structure of Sn-based MHPs called 'grain boundaries' hamper the mobility of charge carriers throughout the material. If used in a TFT, this phenomenon results in a large series resistance that ultimately degrades performance. In addition, a TFT made using an Sn-based MHP arranged into a 3D structure faces a problem of extremely high carrier density of the 3D material, that causes the transistor to be permanently ON unless very high voltages are applied.
Scientists from Tokyo Tech, National Institute for Materials Science and Silvaco Japan have proposed a novel concept based on a hybrid structure for Sn-based metal halide perovskites (MHPs), called the '2D/3D core'shell structure.' In this structure, 3D MHP cores are fully isolated from one another and connected only through short 2D MHP strips (or 'shells'). This alternating arrangement manages to address both these issues, according to the team.
Researchers develop lead-absorbing tapes for sustainable perovskite solar cells
Scientists at the National Renewable Energy Laboratory (NREL) and Northern Illinois University (NIU) have developed a way to prevent lead from escaping damaged perovskite solar cells. This could go a long way in addressing concerns about potential lead toxicity.
 Image by NREL, from Phys.org
Image by NREL, from Phys.org
The light-absorbing layer in perovskite solar cells contains a small amount of lead. Simply encapsulating solar cells does not stop lead from leaking if the device is damaged. Instead, chemical absorption may hold the key. The researchers report being able to capture more than 99.9% of the leakage.
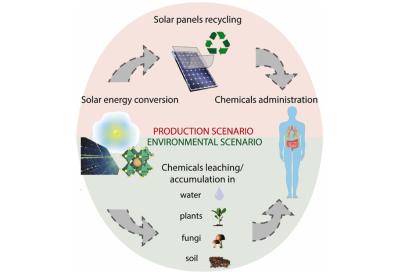
Researchers analyze importance and feasibility of recycling perovskite solar cells
University of Cambridge and Cornell University Researchers have done 'cradle-to-grave' life cycle assessments of a variety of perovskite solar cell architectures, and found that substrates with conducting oxides and energy-intensive heating processes are the largest contributors to primary energy consumption, global warming potential and other types of impact.
The team therefore focus on these materials and processes when expanding to 'cradle-to-cradle' analyses with recycling as the end-of-life scenario. Their results revealed that recycling strategies can lead to a decrease of up to 72.6% in energy payback time and a reduction of 71.2% in greenhouse gas emission factor.
Pagination
- Page 1
- Next page