Pervoskites enable a promising cathode material for low-temperature solid-oxide fuel cells
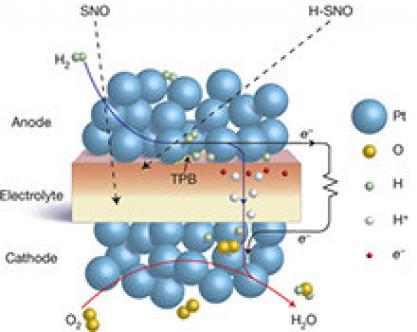
The Australian Nuclear Science and Technology Organisation (ANSTO) has collaborated with researchers at the University of Queensland in Australia, and Shandong University and Nanjing Tech Universities in China on research investigating the possible synergistic effects of a new perovskite cathode material for a low-temperature solid-oxide fuel cell (LT-SOFC) that demonstrates impressive and stable electrochemical performance below 500 °C.
Solid-oxide fuel cells (SOFC) convert the chemical energy in fuel into electricity directly by the oxidation of the fuel. These cells are considered to be highly efficient, exhibit long-term stability, produce low emissions, and are relatively low cost.