Various dangerous chemicals are currently used for agriculture and industry, including fumigants like methyl iodide, which is used to control insects and fungi. The wrong amounts or incorrect use of these fumigants can be harmful to people and degrade the ozone layer. As it’s invisible and doesn’t smell, it’s hard to tell whether there are dangerous amounts of methyl iodide present, and until now the best way to test for it was in a laboratory using expensive, complicated equipment, which isn’t practical in many real-world settings. Some cheaper, lightweight detection methods have been tried, but they didn’t have enough sensitivity and took too long to deliver results.
Now, a research team led by the ARC Centre of Excellence in Exciton Science, along with Australia’s national science agency CSIRO and the Department of Defense, has found a perovskite-based way to detect methyl iodide, with the accuracy, flexibility and speed necessary for practical use. This new sensing mechanism is also versatile enough for use in detecting a wide range of fumigants and chemical warfare agents.
“Perovskite nanocrystals have proved to be a very efficient light emitter,†lead author Dr. Wenping Yin of Monash University said.
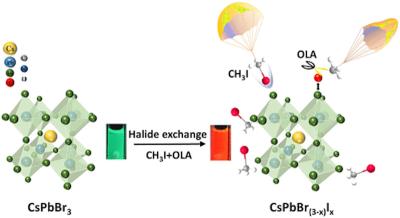
“Here we showed that methyl iodide can react with such perovskites, and do so very quickly following a simple chemical activation step. Critically, this activation step cuts the response time of the sensor from a few hours to just a few seconds.â€
In this process, the ions forming the nanocrystals change quickly when they are exposed to the methyl iodide triggered by a chemical reaction.
The reaction involves exchanging bromide with iodide within the nanocrystal itself, which results in the color change.
Ultimately, the researchers have been able to demonstrate that the change in color is dependent on the perovskite nanocrystal and methyl iodide concentrations.
“Although the chemical mechanism is very complicated, the outcome is just a color change of the light produced by the nanocrystals, which is very easy to detect,†Wenping said.
The new mechanism has the widest range, highest sensitivity and quickest response ever achieved for a technique that doesn’t rely on expensive laboratory instrumentation, producing its results in around five seconds at room temperature.
The researchers now hope their findings will provide a platform for building a test device that can be used in real-world applications.
Senior author Professor Jacek Jasieniak said: “We’ve understood the foundational mechanism for what’s needed to undergo this colorimetric sensing. Now it’s about building a prototype sensing device.
“It needs further development to realize its true potential for broader detection of different types of methyl halide species, as well as pesticides and chemical warfare agents, like teargas, and mustard gas, but the stage is set.â€
Defense scientist and Industry Partner Investigator, Dr. Genevieve Dennison said: “We are very excited about the potential demonstrated by this work and are looking forward to applying the technology to protect our military and first responders.â€