Perovskites are materials that share a crystal structure similar to the mineral called perovskite, which consists of calcium titanium oxide (CaTiO3).
Depending on which atoms/molecules are used in the structure, perovskites can possess an impressive array of interesting properties including superconductivity, ferroelectricity, charge ordering, spin dependent transport and much more. Perovskites therefore hold exciting opportunities for physicists, chemists and material scientists.
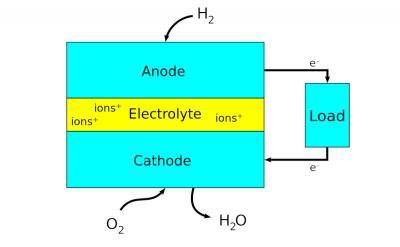
Fuel cells are electrochemical energy conversion devices that produce electricity via chemical reaction. They convert potential chemical energy into electrical energy and generate heat as a by-product. A major advantage of fuel cells is that they are “green” - they generate electricity with very little pollution, as much of the hydrogen and oxygen used in generating electricity ultimately combine to form a harmless byproduct - water.
Fuel cells can be used in a wide range of applications, including transportation, material handling, stationary, portable, and emergency backup power applications. Fuel cells have several benefits over conventional combustion-based technologies currently used in many power plants and passenger vehicles; they can operate at higher efficiencies than combustion engines, and can convert the chemical energy in the fuel to electrical energy with efficiencies of up to 60%. Fuel cells have lower emissions than combustion engines. Hydrogen fuel cells emit only water, so there are no carbon dioxide emissions and negative impact on the environment.
It appears that designing inexpensive, efficient, reliable fuel cells is not such a simple affair.
Scientists have designed many different types and sizes of fuel cells in their search for greater efficiency. A major point is the choice of electrolyte. The design of electrodes, for example, and the materials used to make them, heavily depend on the used electrolyte.
The type of fuel also depends on the electrolyte. Some cells need pure hydrogen, and therefore demand extra equipment such as a "reformer" to purify the fuel. Other cells can tolerate some impurities, but might need higher temperatures to run efficiently. Liquid electrolytes circulate in some cells, which requires pumps. The type of electrolyte also dictates a cell's operating temperature.
Each type of fuel cell has advantages and drawbacks compared to the others, and none of them is currently cheap and efficient enough to widely replace traditional ways of generating power.
Perovskites have been studied for various parts of fuel cells. Components like electrolytes, electrodes and interconnects, have all been targeted as potential beneficiaries of perovskite materials. In SOFCs (solid oxide fuel cells), for example, all components (except for the sealant) can potentially be made of perovskite ceramics. In recent years, a significant amount of time has been invested researching the development of perovskite materials for fuel cells, identifying new mixed conductors and improving the operational performance of existing materials through development of improved cell designs. Hopefully perovskites will be able to improve fuel cell technology so it can be put into widespread use.
The latest Perovskite Fuel Cells news:
Pervoskites enable a promising cathode material for low-temperature solid-oxide fuel cells
The Australian Nuclear Science and Technology Organisation (ANSTO) has collaborated with researchers at the University of Queensland in Australia, and Shandong University and Nanjing Tech Universities in China on research investigating the possible synergistic effects of a new perovskite cathode material for a low-temperature solid-oxide fuel cell (LT-SOFC) that demonstrates impressive and stable electrochemical performance below 500 °C.
Solid-oxide fuel cells (SOFC) convert the chemical energy in fuel into electricity directly by the oxidation of the fuel. These cells are considered to be highly efficient, exhibit long-term stability, produce low emissions, and are relatively low cost.
Surface engineering improves the stability of perovskite-based electrocatalysts for fuel cells
Researchers at MIT tackled the known problem of degradation suffered when perovskite oxides, promising candidates for electrodes in energy conversion devices like fuel cells, are exposed to water or gases such as oxygen or carbon dioxide at elevated temperatures.
The scientists explain that this degradation occurs as the surfaces of these perovskites get covered up by a strontium oxide'related layer, and this layer is insulating against oxygen reduction and oxygen evolution reactions, which are critical for the performance of fuel cells, electrolyzers and thermochemical fuel production. This layer on the electrode surface is detrimental to the efficiency and durability of the device, causing the surface reactions to slow down by more than an order of magnitude.
Perovskites as stable electrolytes to improve fuel cells
Researchers at Purdue University have found that nickel-based perovskites have exceptional properties for use as solid electrolytes in fuel cells. Unlike conventional electrolytes, these nickel-based perovskites are chemically stable in the fuel cell's environment, which could lead to higher performing and longer lasting fuel cells.
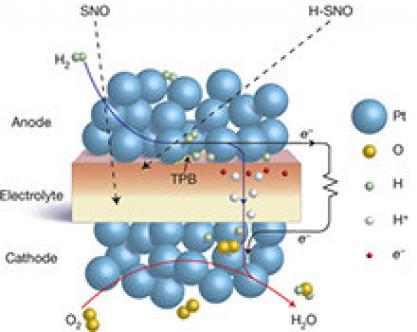 Schematic of the perovskite samarium nickelate (SNO)-electrolyte solid-oxide fuel cell.
Schematic of the perovskite samarium nickelate (SNO)-electrolyte solid-oxide fuel cell.
Solid-oxide fuel cells are considered as one of the most efficient types of fuel cells. They typically use polymers or ceramics as an electrolyte, but finding an effective solid electrolyte'one that conducts protons but blocks electrons'at low operating temperatures of 300'500°C has been a challenge. Most materials, when exposed to low pressure, start to lose oxygen and become electron conductors; The electrolyte separator becomes leaky so it can short circuit the fuel cell or it can start to crack and allow fuel to mix with oxygen.
Pagination
- Previous page
- Page 3