Researchers at Pacific Northwest National Laboratory are evaluating perovskite-structured rare-earth nickelates as alternatives to replace two reactions that are considered a challenge when it comes to electrocatalysts: the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER). Both are important for the development of better fuel cells, metal-air batteries, and electrolytic water-splitting.
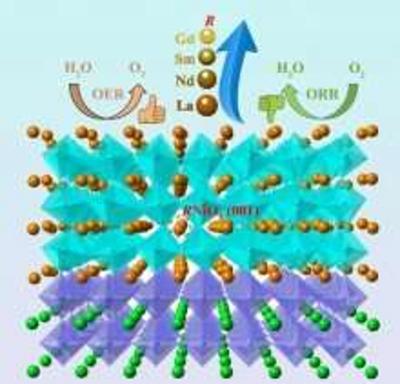
Materials such as platinum, iridium oxide and ruthenium oxide are well suited for these reactions, but they are scarce and expensive. The team has been working to study perovskite-structured rare-earth nickelates (RNiO3) that can serve as bifunctional catalysts capable of performing both OER and ORR.
Recently, the research team conducted performance-testing on a set of well-defined RNiO3 epitaxial thin films and discovered which properties contribute to higher electrocatalytic activity. By tuning the rare earth elements (R), scientists correlated the structural and physical properties of various nickelates with their ORR and OER activities. "We found that tuning the rare earth elements is an effective strategy for balancing ORR and OER activities of bifunctional electrocatalysts".
Designing high-performance bifunctional electrocatalysts requires strategically balancing OER and ORR. By examining these activities in the closely related RNiO3 family, scientists can establish structure-property-performance relationships'an area that has not yet been systematically explored. These fundamental insights can be used to design better, lower-cost catalysts to perform these critical oxygen reactions.
The team investigated a series of rare-earth nickelate thin films grown on SrTiO3(001) by pulsed laser deposition, where R variations include lanthanum (La), neodymium (Nd), samarium (Sm), and gadolinium (Gd). It was found that decreasing the ionic radius of R (rLa > rNd > rSm > rGd) would lead to a decrease in the electronic conductivity of the resulting films, which impacts the ORR in a negative way. On the other hand, the OER activity initially increased upon substituting La with smaller ions, such as Nd or mixtures of Nd and Sm. Reducing the radius of R was shown to increase the average occupancy of the antibonding eg orbital through the formation of oxygen vacancies, a condition known to enhance the OER activity.
The work shows that even though the OER and ORR cannot be enhanced simultaneously in RNiO3, the future design of such bifunctional electrocatalysts should benefit from strategic trade-offs, especially considering the slow kinetics of the OER is the main cause of energy loss for many low-temperature energy storage devices.
The researchers are continuing to investigate the impact of strain and doping on the physical, chemical, and ion-transport properties of RNiO3.



