Researchers develop wearable photoferroelectric perovskite X-Ray detectors
Researchers from China's Shaanxi Normal University, Zhejiang Normal University, China Institute of Radiation Protection and Chinese Academy of Sciences have developed lead-free photoferroelectric hybrid metal halide perovskite flexible membranes for wearable detectors, that offer excellent X-ray response with high sensitivities, low detection limit and impressive imaging capabilities.
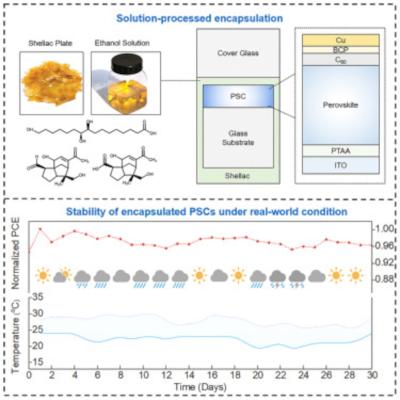
Demonstration and application potential for lead-free photoferroelectric perovskite membrane (LFPPM). a) Optical images of the LFPPM. b) Schematic diagram of LFPPM wearable X-ray dosimeter. c) Schematic diagram of the working principle of wearable X-ray dosimeter. (Image credit: Nanowerk)
High-sensitivity wearable radiation detectors are important for personnel protection in radiation environments such as defense, nuclear facilities, and medical fields. Traditional detectors using bulk crystals tend to lack flexibility. Hybrid metal halide perovskites have shown promise for next-generation radiation detection as they can efficiently absorb high-energy radiation and convert it into electrical signals. However, there are concerns of lead toxicity. More recent efforts have explored lead-free alternatives, but these have generally suffered from poor charge transport properties, reducing their effectiveness as radiation detectors.