Researchers from South Korea have developed a new system that uses perovskite materials for producing hydrogen from water, which they say overcomes several of the common challenges and produces gas more efficiently than other water electrolysis systems.
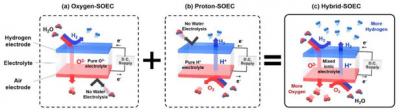
The new device was developed by a research team consisting of scientists from the Ulsan National Institute of Science and Technology (UNIST), Korea Institute of Energy Research (KIER) and Sookmyung Women's University, and is based on an existing design called a solid oxide electrolyzer cell (SOEC). These are similar to other electrolyzers in that an electrical current splits water into its constituent molecules ' hydrogen and oxygen ' which can then be harvested. The difference is that in this setup, both electrodes are solid-state, as is the electrolyte that carries the ions between them.
This is said to have a few advantages over systems that use liquid electrolytes ' namely, the liquids need to be topped up occasionally, and over time they tend to corrode other components. And since solid-state electrolyzers operate at higher temperatures, they don't need as much electrical energy to function because they can draw energy from that heat.
But SOECs still have room for improvement. There are two main designs that use different electrolytes: One allows only oxygen ions to pass through, and the other only hydrogen ions. In both cases, the amount of hydrogen that can be produced is limited.
The researchers developed a new Hybrid-SOEC, which uses a mixed-ion conductor to transport both negatively-charged oxygen ions and positively-charged hydrogen ions (protons) at the same time. The end result had all the benefits of a solid-state electrolyzer, with improved efficiency. "By controlling the driving environment of the hydrogen ion conductive electrolyte, a 'mixed ion conductive electrolyte' in which two ions pass can be realized," says first author of the study. "In Hybrid-SOEC where this electrolyte was first introduced, water electrolysis occurred at both electrodes, which results in significant increase in total hydrogen production."
Using the mixed-ion conductor and electrodes made of layered perovskite, the Hybrid-SOEC produced 1.9 liters (0.5 gal) of hydrogen per hour, running at a cell voltage of 1.5 V and a temperature of 700° C (1,292° F). The researchers say that's four times more efficient than existing water electrolysis systems, and after running the device continuously for 60 hours, there were no signs of that performance degrading.

