Two studies led by teams at the Department of Energy's Oak Ridge National Laboratory have shown that treating a complex oxide crystal with either heat or chemicals caused different atoms to segregate on the surface (surface reconstruction) - creating catalysts with dissimilar behaviors ultimately yielding distinct products. This technique may enable catalyst designers to drive industrially important chemical reactions to improve yields of desired products and reduce unwanted products so post-reaction separation costs can be significantly diminished.
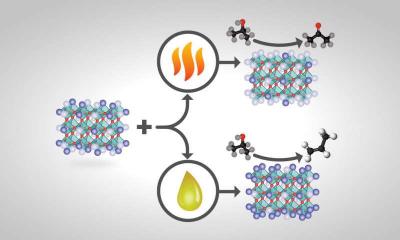
The researchers surveyed four perovskite catalysts. The tests showed that treating a perovskite with heat resulted in a catalyst with more A atoms on its surface, while treating the same perovskite with chemicals instead produced more B atoms on the surface.
The experiments showed a wide range of tunability was possible with different treatments. The same perovskite starting material, subjected to different treatments, could yield a different product.
The researchers plans to go on to explore reconstruction processes of perovskite catalyst surfaces with different termination facets. "The geometry and the composition of the cation and anion [negatively charged ion] are arranged differently when you have different facets," the team explained. "That can give you quite a different chemical reactivity." Also, the researchers are currently expanding their work to tune the surface terminations of perovskites to understand and optimize oxidation and reduction reactions beyond acid-base ones, which could be used in the conversion of shale gas (mostly methane) to valuable chemicals.

