What are Quantum Dots?
Quantum dots (QDs) are semiconducting nanocrystals that are very small ' only a few nanometres in size. In display technologies, the most common types of QDs used are composed of a metal chalcogenide core. These QDs have the chemical formula XY ' where X is a metal and Y is sulfur, tellurium or selenium (e.g. CdTe, CdSe, ZnS) ' which is encased with the shell of a second semiconductor (e.g. CdSe/CdS). Their tiny dimensions mean that charge carriers are confined in close proximity, which gives QDs optical and electronic properties that are substantially different from those of large semiconductor crystals.
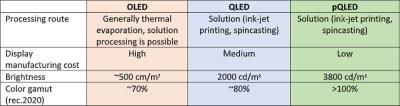
QLEDs vs OLEDs
In particular, QDs have enhanced light absorption and emission, making them particularly suitable for display technologies. Metal chalcogenide quantum dots (MCQDs) have already made it into commercial products ' most notably, in Samsung's QLED television range. Here, a blue LED backlight excites a layer of quantum dots on an LCD panel, causing them to emit light. The color of light emitted by the quantum dots depends on their size ' with small dots emitting blue light, and progressively larger dots emitting green, yellow, orange, and red light.
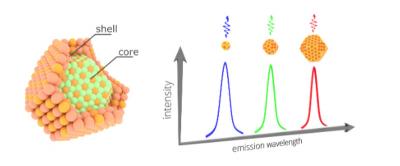 Left: Core-shell quantum dot structure. Right: The size of the dot defines the color of light that the dot emits. (Source: Ossila.com)
Left: Core-shell quantum dot structure. Right: The size of the dot defines the color of light that the dot emits. (Source: Ossila.com)
MCQDs are generally made through solution-based chemical reactions (which is a scalable process), meaning that the dots are compatible with solution-based deposition techniques, such as ink-jet printing. However, the technologies behind OLED and current QLED displays are quite different. QLED displays still require a backlight and LCD to generate light from the QDs - they are 'photo- emissive'. In contrast, the organic materials in OLED displays generate light as a direct response to an electric current ' they are 'electro-emissive'. This means that QLED displays currently cannot achieve the same levels of black as OLED displays, and require extra optical elements (such as polarizers). Photo-emissive QLED displays are currently in development (similar structure to OLED devices, but replacing the organic molecules with QDs), but as of now they are still prohibitively expensive for wide commercialization.
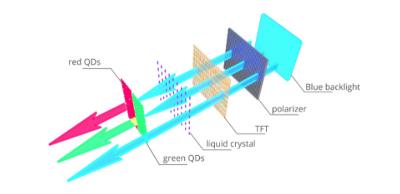 A photo-emissive LCD display using quantum dots to convert the blue backlight emission. (Source: Ossila.com)
A photo-emissive LCD display using quantum dots to convert the blue backlight emission. (Source: Ossila.com)
A new challenger ' Perovskite Quantum Dots (pQDs)
Recent years have seen the emergence of a new type of quantum dot which is simpler and cheaper to manufacture than MCQDs, but still has the much-desired characteristics of high emission efficiency and spectrally-pure emission. They are made of perovskites ' a type of material with atoms arranged in a cubic crystal structure. Perovskites are currently enjoying a surge in popularity, due to their potential application in next-generation solar cells. When perovskite crystals have dimensions of a few nanometres (generally around 4-15 nm for visible light applications), they behave similarly to metal chalcogenide quantum dots.
The most common perovskite quantum dots (pQDs) are called lead halide pQDs. They have the chemical formula APbX 3, where:
- A can be either an organic component (e.g. methylammonium (MA) or formamidinium (FA)) or an inorganic atom, most commonly caesium (Cs).
- X is a halogen (either chlorine (Cl), bromine (Br) or iodine (I)).
The choice of halide affects the emission wavelength, as explained below:
- APbCl 3 dots give blue-like emission
- APbBr 3 dots give green-like emission
- APbI 3 dots give red-like emission
Just like MCQDs, the emission wavelength can be fine-tuned through the dot size, or through creating mixed-halide dots ' where 2 different halogens are present in the same dot. Because they don't contain volatile organic compounds within the dot itself, caesium pQDs tend to be more robust over time compared to MA or FA-based dots.
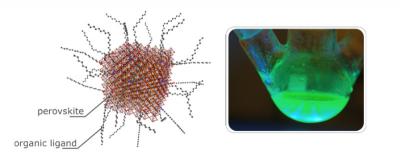 Left: Perovskite quantum dot structure. Right: A solution of CsPbBr 3 pQDs. (Source: Ossila.com)
Left: Perovskite quantum dot structure. Right: A solution of CsPbBr 3 pQDs. (Source: Ossila.com)
Again, a solution-based process is used to create pQDs. This involves mixing perovskite precursor materials with long organic molecules (ligands). These ligands stop the dots from sticking together and passivate the surface, much like the shell on MCQDs. PQDs can either be used as a replacement for MCQDs in photo-emissive displays or can be fabricated into electro-emissive pQLEDs.
The ligands are extremely important when fabricating electro-emissive pQLED devices. Although they prevent the dots from aggregating (which destroys their emission efficiency), the ligands are also electrically insulating and unfortunately prevent electric current flowing through the device (which is needed for electro-emission). Therefore, the number of ligands must be carefully controlled in order to balance emission efficiency with electrical conductivity for efficient light-emitting devices.
The structure of pQLEDs is very similar to that of simple OLEDs, with an emissive pQD layer sandwiched in between electron- and hole-injection layers, which are generally organic molecules or polymers.
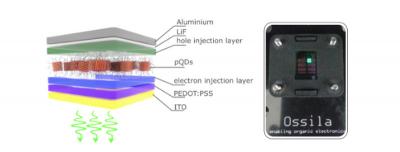 Left: A pQLED device structure. Right: A green pQLED in operation, based on CsPbBr 3 pQDs. (Source: Ossila.com)
Left: A pQLED device structure. Right: A green pQLED in operation, based on CsPbBr 3 pQDs. (Source: Ossila.com)
While pQDs are still in the research phase, the technology is progressing quickly and there have been several demonstrations of bright, efficient electro-emissive pQLEDs. There remain some questions regarding the long-term stability of pQDs, but their low cost, ease of fabrication, and compatibility with current and future manufacturing techniques make them an exciting prospect for the future of display and lighting technologies.
Beyond their use in displays, pQDs have shown promise in photovoltaics. Currently, solar cells fabricated with these nanocrystals have reached an external quantum efficiency of over 13%1. Other potential applications include photodectors, lasers, and single photon sources which are required for quantum cryptography.
References:
1. Enhanced mobility CsPbI3 quantum dot arrays for record-efficiency, high-voltage photovoltaic cells, E. Sanehira et al., Science Advances 27 Oct 2017: Vol. 3, no. 10, eaao4204
 Dr. Dave Coles is a Scientific Content Editor at Ossila. He produces (hopefully) helpful and scientifically accurate content for Ossila.com. After completing his PhD in physics in 2011, Dave held research positions at the University of Oxford and the University of Sheffield, specializing in photonic structures, spectroscopy of organic materials, and a little bit of biophysics. You can find him either on his bike in the Peak District (UK) or in the pub ' or possibly both at the same time.
Dr. Dave Coles is a Scientific Content Editor at Ossila. He produces (hopefully) helpful and scientifically accurate content for Ossila.com. After completing his PhD in physics in 2011, Dave held research positions at the University of Oxford and the University of Sheffield, specializing in photonic structures, spectroscopy of organic materials, and a little bit of biophysics. You can find him either on his bike in the Peak District (UK) or in the pub ' or possibly both at the same time.



