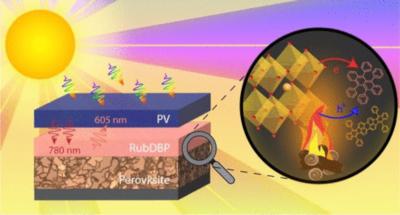
Researchers at Florida State University, the FAMU-FSU College of Engineering, the University of Colorado Boulder and Argonne National Laboratory have studied the effects of two stressors, heat and light, on the triplet generation process at the perovskite/rubrene interface. Following exposure to both stressors, local discrepancies across the upconversion device were discovered. This work emphasizes the challenges and continued potential for the integration of perovskite-sensitized upconversion (UC) into commercial photovoltaic devices.
The first region showed changes to the morphology, and no detectable upconverted emission was observed. Through the combination of optical microscopy and spectroscopy, crystallization of the organic semiconductor layer, degradation of dibenzotetraphenylperiflanthene, and concurrent degradation of the perovskite sensitizer were found. These effects culminate in a reduction in both triplet generation and triplet–triplet annihilation. In the second region, no changes to the morphology were present and visible UC emission was observed following exposure to both stressors. To probe the triplet sensitization process at elevated temperatures, transient absorption spectroscopy was performed. The presence of the excited spin-triplet state of rubrene at 60 °C highlighted successful triplet generation even at elevated temperatures.
Perovskites can enable a phenomenon called upconversion in organic molecules, a process in which the perovskite absorbs low-energy photons and the organic molecules then convert these particles into high-energy photons. “We wondered if there was another way of using the photons — the particles of energy in light — that otherwise would not be converted into electricity?” said Theo Siegrist, a professor in the Department of Chemical and Biomedical Engineering. “We want to store the energy from one photon until a second comes around and combine the two photons into one that can overcome the barrier.”
“The idea is that one usable high energy light particle is emitted again after the upconversion process,” said Lea Nienhaus, assistant professor in the Department of Chemistry and Biochemistry.
Previous research into perovskites examined how high temperatures and light degrade them but,according to the team, hadn’t considered the upconversion process in perovskite/organic bilayers under real-world conditions. Understanding how these devices work in typical heat and light conditions shows researchers where to direct their efforts for implementation into commercial solar cells.
The researchers placed their upconversion devices on a heating element, raising their temperature to about 60 degrees Celsius. Then they used optical spectroscopy and X-ray crystallography to examine their properties.
They found that the performance of the upconversion device was significantly diminished after exposure to high temperatures, but not because of perovskite degradation. Instead, the organic molecules required for the upconversion process crystallized under the heat, rendering the device ineffective.
“The perovskite on its own, if you heat it and shine light on it, it degrades,” Niehaus said. “As soon as you put the organic molecules on top, it no longer degrades. The organic molecules are contributing to a more long-lived perovskite, which I think is a very useful result. There are still engineering issues to be solved to make perovskite-based upconversion devices viable, but our hope is that this work is part of addressing those issues.”