A research team from the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) in Warsaw and the Ecole Polytechnique Federale de Lausanne (EPFL) in Lausanne (Switzerland), co-operating within the GOTSolar project, has demonstrated a perovskite solar cell with a significantly smaller number of structural defects. The unexpected improvement of the photovoltaic performance was observed when perovskites produced by mechanochemistry were used for the construction of a typical photovoltaic cell.
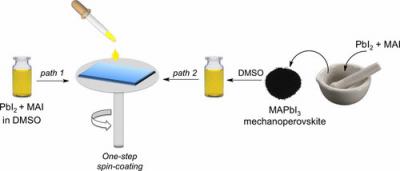
The group from IPC PAS (Warsaw University of Technology) was the first to demonstrate that polycrystalline halide perovskites (CH3NH3)PbI3 can be produced in mechanochemical reactions. Recently, the group presented the mechanochemical production of mixed perovskites, those in which several different types of ions alternate in position A. This is an important achievement, because by carefully altering the chemical composition of the perovskite materials, they can be adapted to specific applications in photovoltaics, catalysis and other fields of science and technology.
"In practice, the mechanochemical production of our perovskites is as follows. Two powders, e.g. white methylammonium iodide CH3NH3I and yellow lead iodide PbI2, are placed in a mill equipped with a few steel balls. Then, we grind them for several dozen minutes, producing a homogeneous black powder of the perovskite (CH3NH3)PbI3, which can be directly used for the construction of photovoltaic cells. You do not need to use high temperature, organic solvents, or worry about waste. The whole process is really fast and efficient. It is green chemistry," says a PhD student at IPC PAS.
The materials produced here are assembled and tested at EPFL in collaboration with a world-class Swiss chemist, Prof. Michael Graetzel. Interestingly, in these jointly developed cells, a perovskite layer of around 300 nanometers was sufficient. In the future, such thin coverage will reduce the unit cost of cell production.
"An important property characterizing the quality of a photovoltaic cell is the amount of electrical charge accumulating at the boundary of individual cell layers. If there is too much, the cell undergoes degradation more rapidly. Perovskites obtained by mechanochemistry formed a very homogeneous layer, which reduced the number of defects in the structure, impairing the work of the cell, and reduced the amount of charge deposited on the surface," says the team.
Researchers have yet to figure out why the electrical properties of perovskite cells obtained by grinding are better. It seems that the solvent used in production of perovskites plays a negative role. It can be incorporated into the structure of the material, and upon deposition of the perovskite on the substrate, its residues may cause the formation of defects in the crystalline structure. Microscopic and electrical studies have shown that higher-quality mechanochemically synthesized perovskites (obtained without solvents) are associated with reducing the number of traps for charge carriers formed at the junction of the material with the substrate.

