Florida State University (FSU) researchers are working to better understand the fundamental processes in perovskites. As art of this task, they found that small tweaks to the chemical makeup of the materials as well as the magnitude of the electrical field it is exposed to can greatly affect the overall material stability.
"How can we make perovskites more stable under real-world conditions in which they'll be used?" FSU Assistant Professor of Chemistry and Biochemistry Lea Nienhaus said. "What is causing the degradation? That's what we're trying to understand. Perovskites that don't degrade quickly could be a valuable tool for obtaining more energy from solar cells."
In their recent work, the researchers investigated the combined influence of light and elevated temperature on the performance of mixed-cation mixed-halide perovskites.
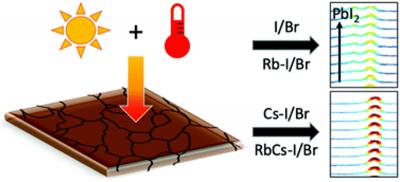
They found that adding a small amount of the element cesium to the perovskite film increases the stability of the material under light and elevated temperatures. Adding rubidium, on the other hand, led to worse performance.
"We found that depending on the choice of the cation, two pathways of degradation can be observed in these materials, which we then correlated to a decrease in performance," said Sarah Wieghold, former FSU postdoctoral researcher and now an assistant scientist at the Center for Nanoscale Materials and the Advanced Photon Source at Argonne National Laboratory. "We also showed that the addition of cesium increased the film stability under our testing conditions, which are very promising results."
They also found that a decrease in film performance for the less stable perovskite mixtures was correlated with the formation of the compound lead bromide/iodide and an increase in electron-phonon interactions. The formation of lead bromide/iodide is due to the unwanted degradation mechanism, which needs to be avoided to achieve long-term stability and performance of these perovskite solar cells.
The team also explored the link between voltage and the performance of perovskite materials. This showed that the ion movement in the material changes the underlying electrical response, which will be a critical factor in the photovoltaic performance.
"Perovskites present a great opportunity for the future of solar cells, and it's exciting to help move this science forward," Nienhaus said.

